Unique KENCOS Technology
KENCOS produces molecular hydrogen through the electrolysis process.
Electrolysis is the process of using electricity to split water into hydrogen and oxygen. This process takes place inside an electrolyzer.
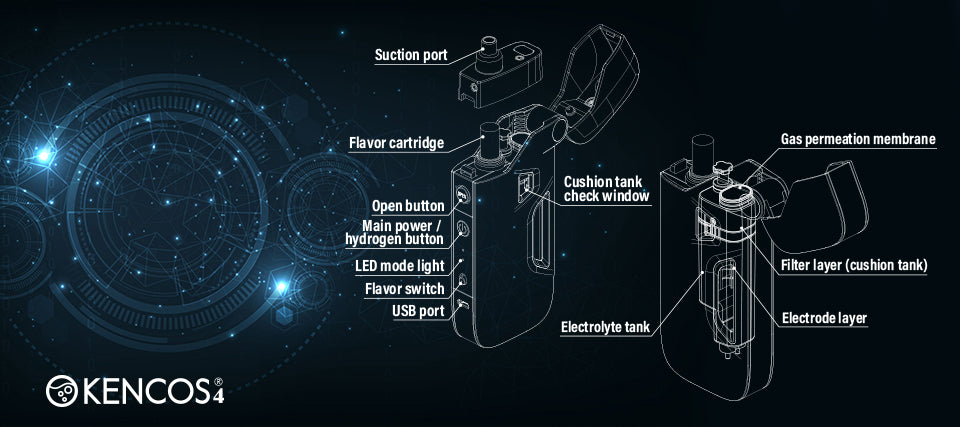
How does KENCOS produce hydrogen?
Inside the KENCOS tank are electrodes made of platinum and titanium. These carry the electrical current and deliver them to the specially formulated liquid electrolysis solution. This solution contains sodium citrate and pure water. Sodium citrate is simply there to act as a conductor, attracting electrical current, which in turn splits pure water into 66.6% hydrogen and 33.3% oxygen. As you breathe through the mouthpiece of the unit, you are directly breathing this gas into your lungs.
Electrolysis is the most practical way to produce hydrogen in inhalers, as it avoids the need to store excess gases and simply allows the user to inhale the hydrogen as it is produced.

A common question we get is “Why can't I just use tap water to generate the hydrogen instead of a specially formulated solution? ”
The answer to this is that tap water will certainly work to achieve electrolysis, however the danger with this is that tap water is not purified and will be contaminated, adding harmful contaminations to the gas you inhale.
Pure water does not conduct electricity, so the electrolysis solution KENCOS uses is pure water with only a small amount of sodium citrate to conduct electrical current.

